Gas pressure is the pressure of the gas giver wall, which is the macroscopic manifestation of a large number of molecules continuously impacting the wall and is an important parameter used to describe the state of the system. Many physical and chemical properties, such as point, boiling point, vapor pressure, are almost all pressure-dependent. Pressure is also an important factor in the study of chemical thermodynamics and chemical kinetics. Therefore, the measurement of pressure is of great significance.
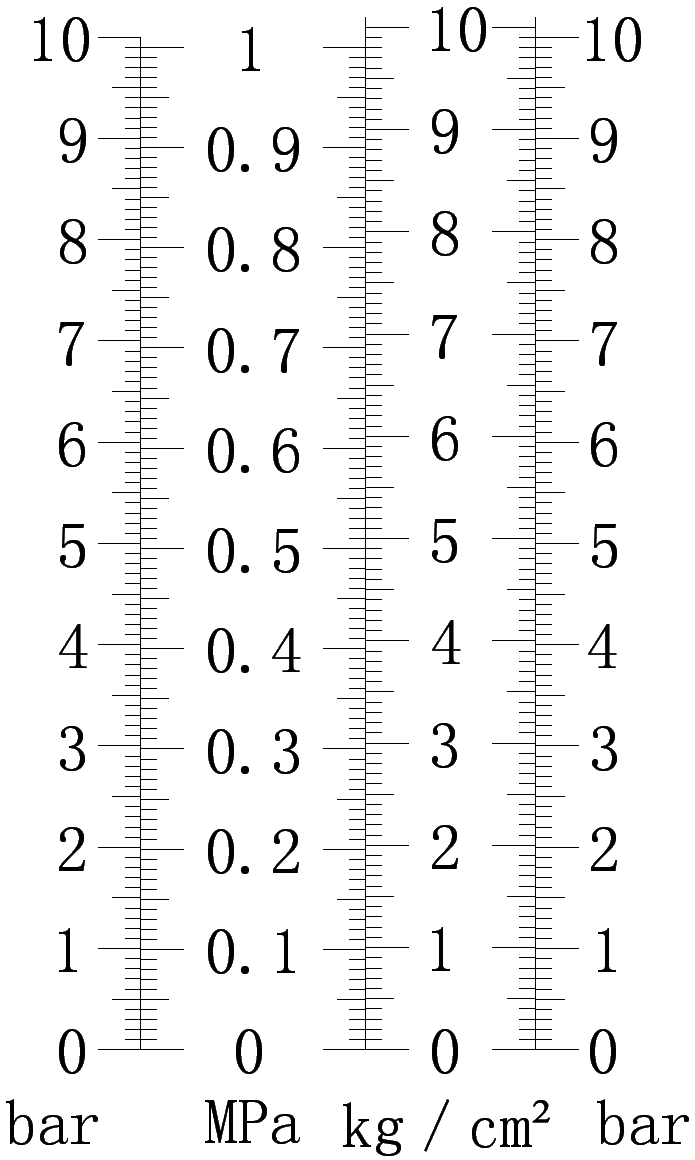
Commonly used units are: bar (bar), pascal (Pa). A unit of pressure, in physics, refers to the force acting vertically on the surface of an object. The unit is Pascal (abbreviated as Pa The letter is “Pa”). (Strictly speaking, the unit of pressure should be Newton N.) The unit of pressure is Pascal, and it is customary to call pressure pressure in life) In China, we generally describe the pressure of gas as “kilogram” (not “jin”), the unit “kg•f/cm2″, is a kilogram of pressure is a kilogram of force acting on a square centimeter.
1Standard atmospheric pressure = 760mmHg = 76cmHg = 1.01325×105Pa=10.336m water column. 1 standard atmospheric pressure
= 101325 N/㎡. (Usually 1 standard atmosphere = 1.01×105Pa in calculations)
If you want to do an exact calculation, the relationship is as follows:
Pressure conversion relationship:
1 dyne per square centimeter (dyn/cm2) = 0.1 Pa (Pa)
1 torr = 133.322 Pa
1 millimeter of mercury (mmHg) = 133.322 Pa (Pa)
1 mm water column (mmH2O) = 9.80665 Pa (Pa)
1 engineering atmospheric pressure = 98.0665 kilopascals (kPa)
1 kilopascal (kPa) = 0.145 pound forces per square inch (psi) = 0.0102 kilogram force per square centimeter (kgf/cm2) = 0.0098 atm (atm)
1 pound force per square inch (psi) = 6.895 kilopascals (kPa) = 0.0703 kilogram force per square centimeter (kgf/cm2) = 0.0689 bar (bar) = 0.068 atm (atm)
1 Physical atmospheric pressure (atm) = 101.325 kilopascals (kPa) = 14.695949400392 pounds force per square inch (psi) = 1.01325 bar (bar)
|
Standard comparison table of common pressure units |
Post time: Feb-27-2023